Abstract
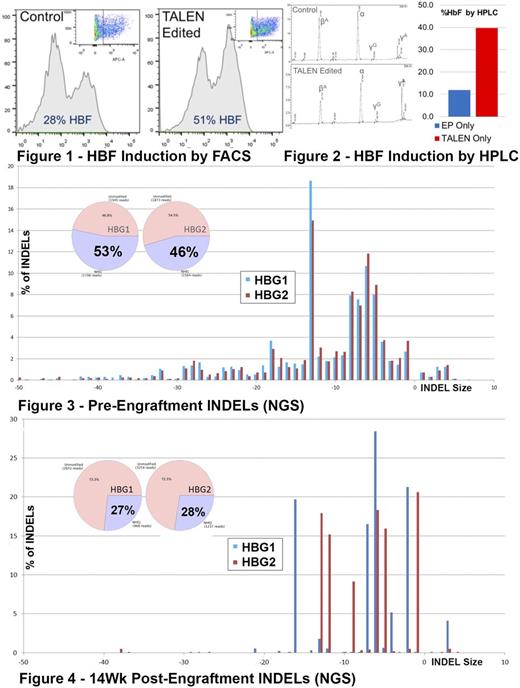
Hemoglobinopathies including sickle cell disease (SCD) and β-thalassemia are the most common single-gene disorders in the world and represent a major global public health concern. The unifying principle of this heterogeneous mix of gene mutations is the decreased production of wild type hemoglobin molecules either due to structural defects in the case of SCD or insufficient production of β-hemoglobin subunits. Hemoglobinopathy patients with gene variants that induce expression of fetal (γ) hemoglobin often exhibit a less severe phenotype. Recent evidence has demonstrated that the in vitro introduction of a previously described naturally occurring 13bp deletion in the promoter of the Aγ globin gene can reconstitute the induction of fetal hemoglobin seen in patients with this mutation. As temporary expression of endonuclease is a key element of a clinically viable editing technique, we have generated a set of TALENs (Transcription Activator-Like Effector Nucleases) targeting the 13bp deletion site that are transfected as mRNA. In human CD34 cells, transfection with and expression of the TALEN pair results in INDEL generation at both the Aγ and Gγ globin loci confirmed by sequencing, T7 analysis and ddPCR. INDEL generation occurs in both the Aγ and Gγ globin promoters with slightly higher rates at the Aγ globin locus (~53%) compared to the Gγ globin locus (~45%) (Figure 3). Compared to control cells, edited CD34+ cells placed in erythroid differentiation media result in an increased expression of fetal hemoglobin with a near doubling in the frequency of 'F-Cells' by flow cytometry and a threefold increase in γ globin protein detected by HPLC (Figure 1 & 2). These findings support a model in which TALEN induced targeted INDELs in the γ globin promoter are capable of de-repressing fetal hemoglobin in hematopoietic progenitor cells. d13 TALEN edited CD34 cells have also been transplanted into NSG-W41 mice, a strain demonstrated to be more permissive of human erythropoiesis and are capable of high rates of engraftment with minimal conditioning (66-95% human chimerism at 14 weeks). An analysis of the INDEL rates retained by the engrafted cell populations 14 weeks post-transplantation demonstrates an average gene editing retention rate of approximately 50% (individual animals ranged from 15-77%) (Figure 4). These edits are retained in multiple hematopoietic cell lineages from each mouse suggesting that editing is occurring at least in the hematopoietic progenitor population and does not rule out the possibility of edited long term repopulating stem cells. A unique feature of targeted gene editing at this locus is that therapeutic benefit can be theoretically derived both from INDEL generation as well as the introduction of synthetic sequence via the homologous repair pathway. AAV transfected homologous recombination templates have been generated with a goal of further manipulating hemoglobin expression and allowing for the selection of edited cells. A basic GFP selection template has been successfully integrated at the target site (approximately 27% efficiency) resulting in erythroid and myeloid methylcellulose progenitor colonies detectable by fluorescence microscopy. Other repair templates that either introduce the specific 13bp deletion γ-hemoglobin promoter or elements of the β-hemoglobin promoter result in higher rates of HbF expression in GFP+ cells. A T87Q anti-sickling β hemoglobin construct has also been successfully integrated and expressed in human CD34 cells resulting in both an increase in HbF expression as well as the presence of T87Q hemoglobin making up a combined 60% of the total β hemoglobin proteins expressed in edited cells. Continued efforts are underway to optimize repair template designs, editing efficiencies and selection strategies to prepare this approach for clinical applications.
Scharenberg: Casebia: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal